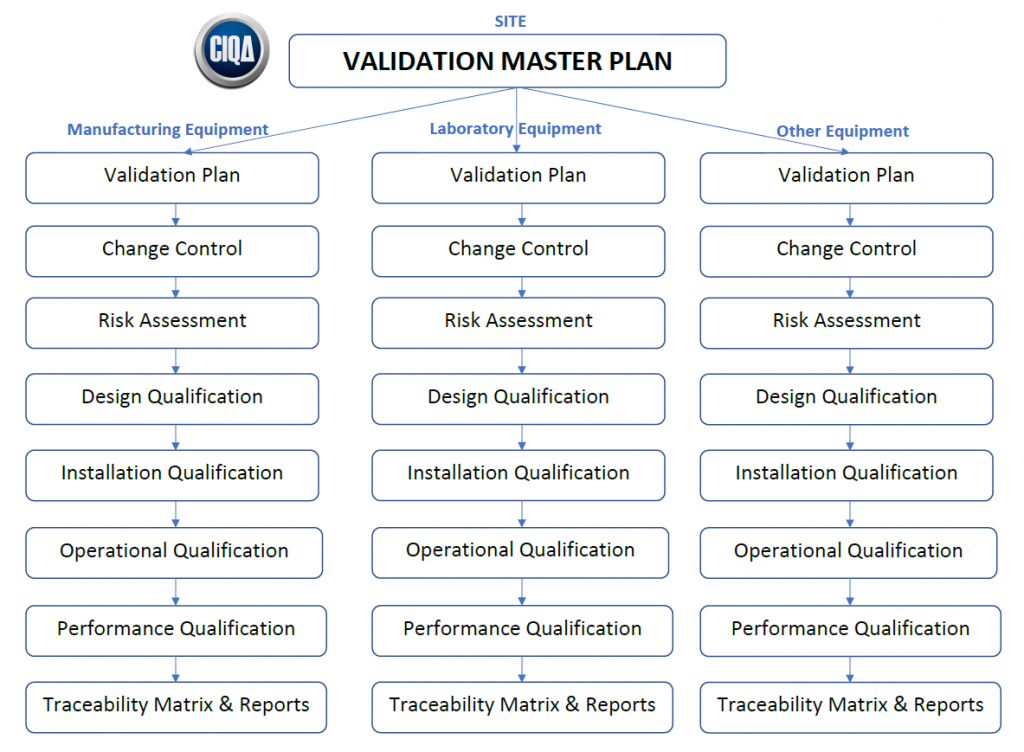
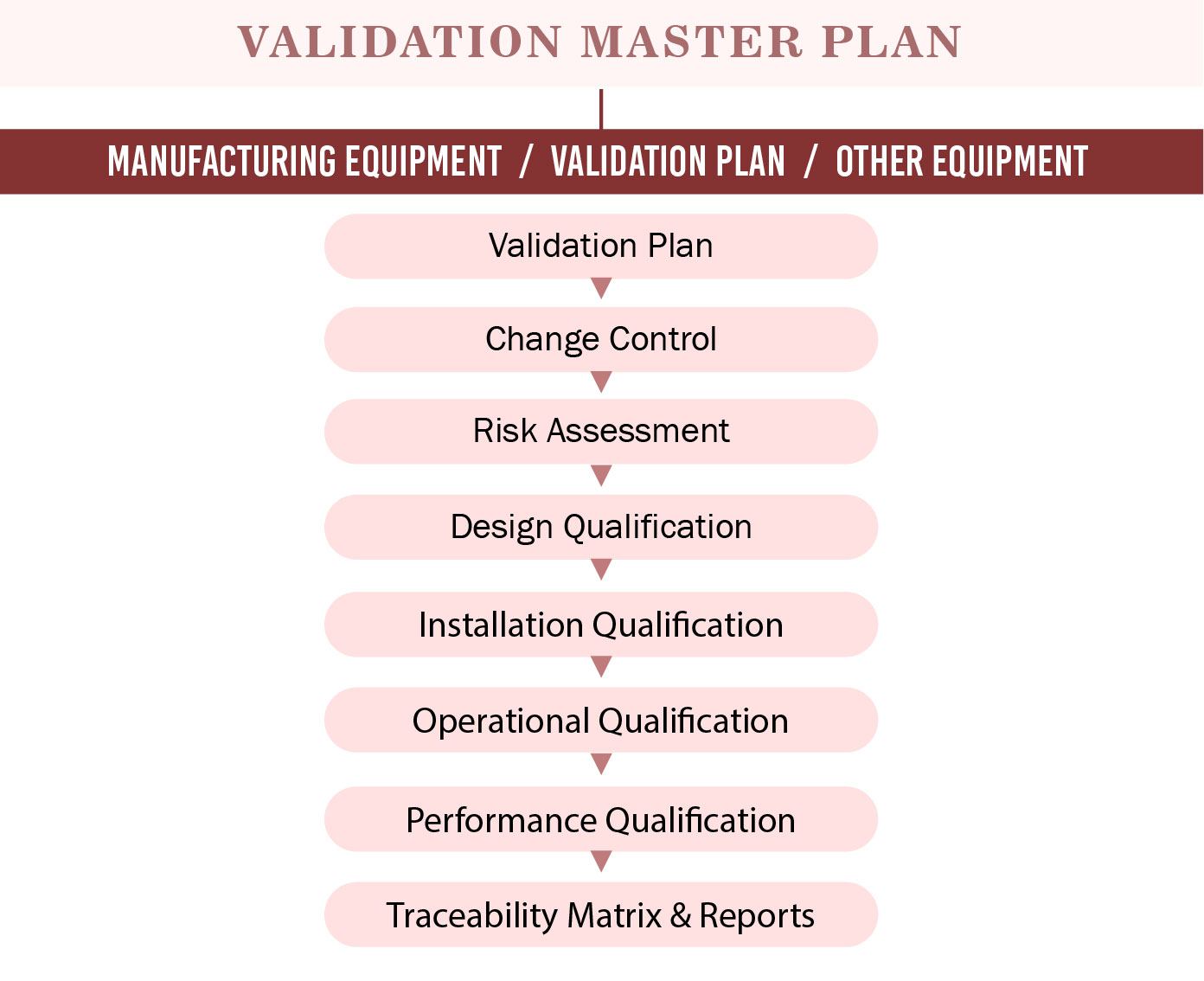
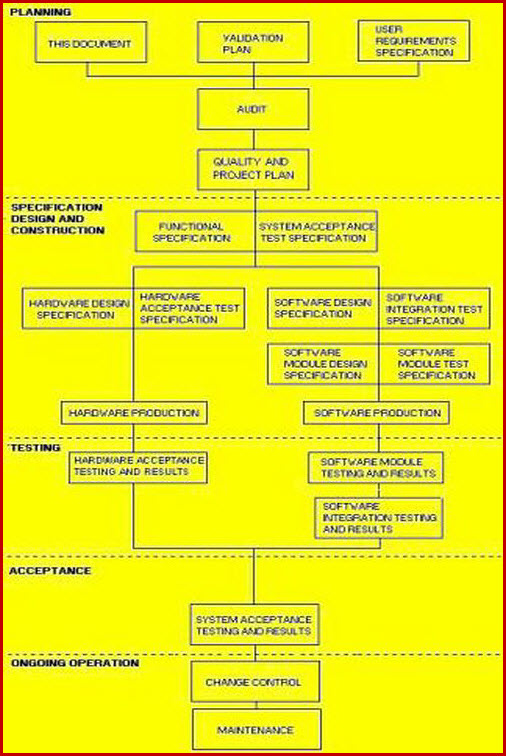
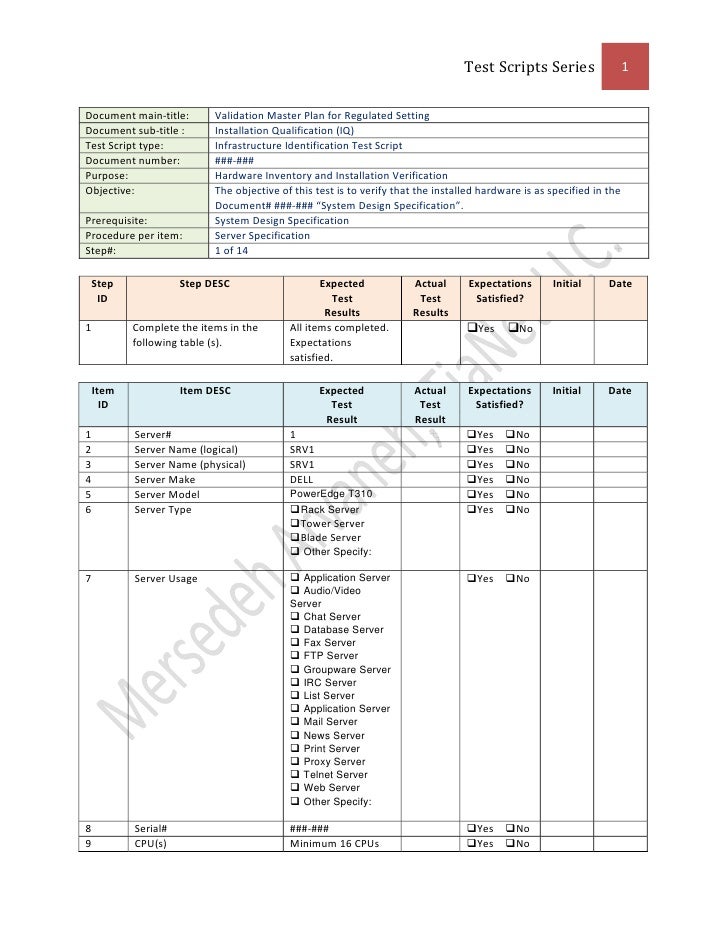
Validation Master Plan Template - Web validation master plan examples. Web the purpose of the validation master plan template (vmp) is to describe the organization’s overall strategy, approach, and responsibilities for. Web the validation master plan is designed to provide a planned and systematic framework within which all validation activities will occur. Web three (3) options to create a validation master plan. If you let me know what is the. Purpose the purpose of this guideline is to provide guidance on the preparation of validation master plans (vmp). I am attaching a file concening the validation master plan and its design. The validation master plan includes: Scope and applicability all functions,. Web use this template to:
How to create a Validation Master Plan in 5 steps. Templates & more
Systems, equipment, methods, facilities, etc., that. Web pharmacy manufacturing unit validation master plan (vpm). Web what is a validation master plan? If you let me know what is the. Web three (3) options to create a validation master plan.
Validation Master Plan. Understand the importance and benefits
Web the validation master plan includes: Web the purpose of this validation master plan is to define the scope of necessary activities to successfully validate the new facilities,. Web validation master plan examples. Select to establish a validation master map previous the next fda audit? The validation master plan includes:
FREE 9+ Sample Validation Plan Templates in PDF MS Word
Web the validation master plan is designed to provide a planned and systematic framework within which all validation activities will occur. Web the validation master plan includes: Web the purpose of the validation master plan template (vmp) is to describe the organization’s overall strategy, approach, and responsibilities for. Select to establish a validation master map previous the next fda audit?.
Medical Device Validation Master Plan (VMP) Consultancy Firm Operon
Select to establish a validation master map previous the next fda audit? Web pharmacy manufacturing unit validation master plan (vpm). Scope and applicability all functions,. Web the validation master plan is designed to provide a planned and systematic framework within which all validation activities will occur. Web background the need for revision of the published world health organization (who) supplementary.
Validation Master Plan FDA EU WHO cGMP QbD FLCV SOP's
Web a validation master plan (vmp) outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated,. Web the validation master plan is designed to provide a planned and systematic framework within which all validation activities will occur. Web what is a validation master plan? Systems, equipment, methods, facilities, etc., that are in.
Master Validation Plan
Web the validation master plan is designed to provide a planned and systematic framework within which all validation activities will occur. Web the purpose of the validation master plan template (vmp) is to describe the organization’s overall strategy, approach, and responsibilities for. Web three (3) options to create a validation master plan. Web this template describes the information that needs.
Validation Master Plan
Web the validation master plan includes: Web what is a validation master plan? Web this template describes the information that needs to be presented in a validation master plan and provides examples. Web this document comprises individual recommendations on four topics relating to equipment qualification and process. Systems, equipment, methods, facilities, etc., that are in the scope of the.
Validation Master Plan Template Verification And Validation
Web the purpose of this validation master plan is to define the scope of necessary activities to successfully validate the new facilities,. Web what is validation master plan (vmp): Web a validation master plan (also referred to as the vmp) is a document which outlines the principles tied to the qualification of a. Web validation master plan examples. Web this.
Validation Master Plan Template Validation Center
Web the purpose of the validation master plan template (vmp) is to describe the organization’s overall strategy, approach, and responsibilities for. Web a validation master plan (also referred to as the vmp) is a document which outlines the principles tied to the qualification of a. Web the validation master plan is designed to provide a planned and systematic framework within.
Validation Master Plan
Web three (3) options to create a validation master plan. Web a validation master plan (also referred to as the vmp) is a document which outlines the principles tied to the qualification of a. Systems, equipment, methods, facilities, etc., that. Web this document comprises individual recommendations on four topics relating to equipment qualification and process. Select to establish a validation.
Web what is validation master plan (vmp): Web the validation master plan is designed to provide a planned and systematic framework within which all validation activities will occur. Web the purpose of this validation master plan is to define the scope of necessary activities to successfully validate the new facilities,. Web 16 operational qualification challenge process parameters to assure the process will result in product that meets. Web the purpose of the validation master plan template (vmp) is to describe the organization’s overall strategy, approach, and responsibilities for. Systems, equipment, methods, facilities, etc., that. The validation master plan serves as a roadmap that helps to set the. Purpose the purpose of this guideline is to provide guidance on the preparation of validation master plans (vmp). Web use this template to: Web this document comprises individual recommendations on four topics relating to equipment qualification and process. Web a validation master plan (also referred to as the vmp) is a document which outlines the principles tied to the qualification of a. Scope and applicability all functions,. Web three (3) options to create a validation master plan. I am attaching a file concening the validation master plan and its design. Systems, equipment, methods, facilities, etc., that are in the scope of the. Web pharmacy manufacturing unit validation master plan (vpm). Web a validation master plan (vmp) outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated,. The validation master plan includes: Web this template describes the information that needs to be presented in a validation master plan and provides examples. Web validation master plan template document control details this will include details such as vmp reference number, version.
Web A Validation Master Plan (Vmp) Outlines The Principles Involved In The Qualification Of A Facility, Defining The Areas And Systems To Be Validated,.
Systems, equipment, methods, facilities, etc., that are in the scope of the. Web this template describes the information that needs to be presented in a validation master plan and provides examples. Web the validation master plan is designed to provide a planned and systematic framework within which all validation activities will occur. The validation master plan includes:
Web What Is Validation Master Plan (Vmp):
Scope and applicability all functions,. Web the purpose of the validation master plan template (vmp) is to describe the organization’s overall strategy, approach, and responsibilities for. Web 16 operational qualification challenge process parameters to assure the process will result in product that meets. Web background the need for revision of the published world health organization (who) supplementary guidelines on good.
Web Validation Master Plan Template Document Control Details This Will Include Details Such As Vmp Reference Number, Version.
Web the purpose of this validation master plan is to define the scope of necessary activities to successfully validate the new facilities,. I am attaching a file concening the validation master plan and its design. Web the validation master plan includes: Select to establish a validation master map previous the next fda audit?
Web A Validation Master Plan (Also Referred To As The Vmp) Is A Document Which Outlines The Principles Tied To The Qualification Of A.
Web pharmacy manufacturing unit validation master plan (vpm). The validation master plan serves as a roadmap that helps to set the. If you let me know what is the. Systems, equipment, methods, facilities, etc., that.