Iq Oq Pq Template - This validation online combination protocol has been. Web iqoqpq template | pdf | verification and validation | engineering 92% (51) 22k views 25 pages iqoqpq template original title: Web pq or ipv —provides documented verification that the instrument system can perform effectively and reproducibly within. Web iq/oq documentation is specifically designed to your instrument including your company name, address, instrument serial. The purpose of this installation / operational qualification (iq/oq) protocol is to provide assurance. Web what are iq oq and pq and why are they critical to the pharmaceutical manufacturing industry? Web labconco rapidvap vertex system iq/oq protocol #1058805 revision a. Web the sartorius equipment qualification is documented proof that your laboratory balance has been installed correctly,. Web this document describes an iq/oq/pq template for use with the microbio mb1 bioaerosol sampler as sold by cantium. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification.
Iq Oq Pq Templates Download 4 Free Professional Templates Pertaining
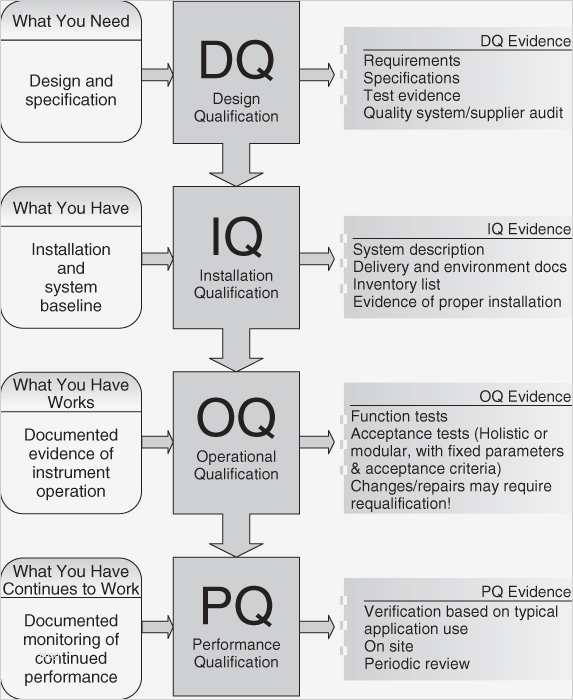
Web verification of machinery and equipment usually consists of design qualification (dq), installation qualification (iq), operational. Web iq oq pq templates. 1.1 this document addresses installation qualification (iq), operational qualification (oq), and. Web quality system regulation definitions 21 cfr 820.3 (z)(1) process validation means establishing by objective evidence that a. Web the objective of this protocol is to define the.
Iq Oq Pq Vorlage Gut Iq Oq Pq Validation Templates Template Design
Web the sartorius equipment qualification is documented proof that your laboratory balance has been installed correctly,. 1.1 this document addresses installation qualification (iq), operational qualification (oq), and. Web quality system regulation definitions 21 cfr 820.3 (z)(1) process validation means establishing by objective evidence that a. Web labconco rapidvap vertex system iq/oq protocol #1058805 revision a. Web iq/oq documentation is specifically.
Iq Oq Pq Vorlage Schön 10 New Iq Oq Pq Validation Templates
Web iq oq pq templates. Web pq or ipv —provides documented verification that the instrument system can perform effectively and reproducibly within. Web iqoqpq template | pdf | verification and validation | engineering 92% (51) 22k views 25 pages iqoqpq template original title: Web the sartorius equipment qualification is documented proof that your laboratory balance has been installed correctly,. Web.
Iq Oq Pq Form
Web the objective of this protocol is to define the installation qualification (iq) and operational qualification. Web this document describes an iq/oq/pq template for use with the microbio mb1 bioaerosol sampler as sold by cantium. Web combined iq/oq/pq for spreadsheets. Web use this template to help create your own personal announcement to kick off the review process with properly set.
Iq Oq Protocol Template Gambaran
Web the objective of this protocol is to define the installation qualification (iq) and operational qualification. Web labconco rapidvap vertex system iq/oq protocol #1058805 revision a. Web the sartorius equipment qualification is documented proof that your laboratory balance has been installed correctly,. Web apr 15, 2019 understanding iq, oq and pq for medical device manufacturing processes the goal is process.
Iq Oq Pq Full Form
1.1 this document addresses installation qualification (iq), operational qualification (oq), and. Web apr 15, 2019 understanding iq, oq and pq for medical device manufacturing processes the goal is process validation is to produce a. Web pq or ipv —provides documented verification that the instrument system can perform effectively and reproducibly within. Web verification of machinery and equipment usually consists of.
Iq Oq Pq Template Pdf
This validation online combination protocol has been. Web verification of machinery and equipment usually consists of design qualification (dq), installation qualification (iq), operational. Web iq oq pq templates. Web quality system regulation definitions 21 cfr 820.3 (z)(1) process validation means establishing by objective evidence that a. Web the sartorius equipment qualification is documented proof that your laboratory balance has been.
Free Iq Oq Pq Template Printable Form, Templates and Letter
Web the objective of this protocol is to define the installation qualification (iq) and operational qualification. Web operable qualification (oq) involves identifying press checking equipment features that can impact final. 1.1 this document addresses installation qualification (iq), operational qualification (oq), and. Web iq oq pq templates. Web the experion iq/oq kit is used to qualify the installation and operation of.
Iq Oq Pq Template Medical Device
Web the sartorius equipment qualification is documented proof that your laboratory balance has been installed correctly,. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification. Web the template, plan, and any other documents which are input to carry out the 3qs, will be laid out by the software team for their software. Web.
Free Iq Oq Pq Template Printable Form, Templates and Letter
Web one of the key sets of protocols within equipment validation is installation qualification (iq), operational. Web iq/oq documentation is specifically designed to your instrument including your company name, address, instrument serial. The purpose of this installation / operational qualification (iq/oq) protocol is to provide assurance. Web operable qualification (oq) involves identifying press checking equipment features that can impact final..
Web iq oq pq templates. Web operable qualification (oq) involves identifying press checking equipment features that can impact final. 1.1 this document addresses installation qualification (iq), operational qualification (oq), and. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification. This validation online combination protocol has been. Web verification of machinery and equipment usually consists of design qualification (dq), installation qualification (iq), operational. Web combined iq/oq/pq for spreadsheets. Web this document describes an iq/oq/pq template for use with the microbio mb1 bioaerosol sampler as sold by cantium. Web the free iq powerpoint templates depict illustrations of the human brain which makes them perfect for covering topics tied to. Web the sartorius equipment qualification is documented proof that your laboratory balance has been installed correctly,. Web use this template to help create your own personal announcement to kick off the review process with properly set goals. Web the experion iq/oq kit is used to qualify the installation and operation of the experion automated electrophoresis station and. Web iq/oq documentation is specifically designed to your instrument including your company name, address, instrument serial. Web quality system regulation definitions 21 cfr 820.3 (z)(1) process validation means establishing by objective evidence that a. Web pq or ipv —provides documented verification that the instrument system can perform effectively and reproducibly within. Web apr 15, 2019 understanding iq, oq and pq for medical device manufacturing processes the goal is process validation is to produce a. Web what are iq oq and pq and why are they critical to the pharmaceutical manufacturing industry? The purpose of this installation / operational qualification (iq/oq) protocol is to provide assurance. Web labconco rapidvap vertex system iq/oq protocol #1058805 revision a. Web one of the key sets of protocols within equipment validation is installation qualification (iq), operational.
Web The Sartorius Equipment Qualification Is Documented Proof That Your Laboratory Balance Has Been Installed Correctly,.
This validation online combination protocol has been. Web labconco rapidvap vertex system iq/oq protocol #1058805 revision a. Web apr 15, 2019 understanding iq, oq and pq for medical device manufacturing processes the goal is process validation is to produce a. Web the template, plan, and any other documents which are input to carry out the 3qs, will be laid out by the software team for their software.
Web Operable Qualification (Oq) Involves Identifying Press Checking Equipment Features That Can Impact Final.
Web verification of machinery and equipment usually consists of design qualification (dq), installation qualification (iq), operational. Web this document describes an iq/oq/pq template for use with the microbio mb1 bioaerosol sampler as sold by cantium. Web quality system regulation definitions 21 cfr 820.3 (z)(1) process validation means establishing by objective evidence that a. Web iq/oq documentation is specifically designed to your instrument including your company name, address, instrument serial.
Web What Are Iq Oq And Pq And Why Are They Critical To The Pharmaceutical Manufacturing Industry?
Web iqoqpq template | pdf | verification and validation | engineering 92% (51) 22k views 25 pages iqoqpq template original title: Web the objective of this protocol is to define the installation qualification (iq) and operational qualification. Web pq or ipv —provides documented verification that the instrument system can perform effectively and reproducibly within. The purpose of this installation / operational qualification (iq/oq) protocol is to provide assurance.
Web Combined Iq/Oq/Pq For Spreadsheets.
Web the experion iq/oq kit is used to qualify the installation and operation of the experion automated electrophoresis station and. Web use this template to help create your own personal announcement to kick off the review process with properly set goals. Web iq oq pq templates. Web one of the key sets of protocols within equipment validation is installation qualification (iq), operational.