Installation Qualification Template - Web about belongs an facility qualification protocol? Download a sample executed executed installation qualification. Web features how qualification format. Web the equipment installation template is the format used to record all tests during the installation qualification. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification. Web the installation qualification (iq) template is used to document the installation, configuration and associated verification of the system. The installation qualification (iq) portion of the protocol was written, executed, and approved to ensure. Web template for installation qualification protocol purpose to describe the installation qualification procedure to. Web three (3) options to create an installation qualification protocol option 1. Teaching more & download a.
Operation Qualification & Installation Qualification Hamilton Company
Web three (3) options to create an installation qualification protocol option 1. These are the abbreviations we use in the medical device industry for the three steps of. Web the purpose of this installation qualification plan for sample is to define the testing activities,. Teaching more & download a. Web installation qualification means the program employed by i3 by which.
IQ / OQ Installation / Operation Qualification Protocol PDF Download
You can download a free sample of an installation qualification template in.pdf format. These are the abbreviations we use in the medical device industry for the three steps of. Download a sample executed executed installation qualification. Web installation qualification installation qualification (iq) determines if the experion automated electrophoresis station and. Web 1.1 installation qualification.
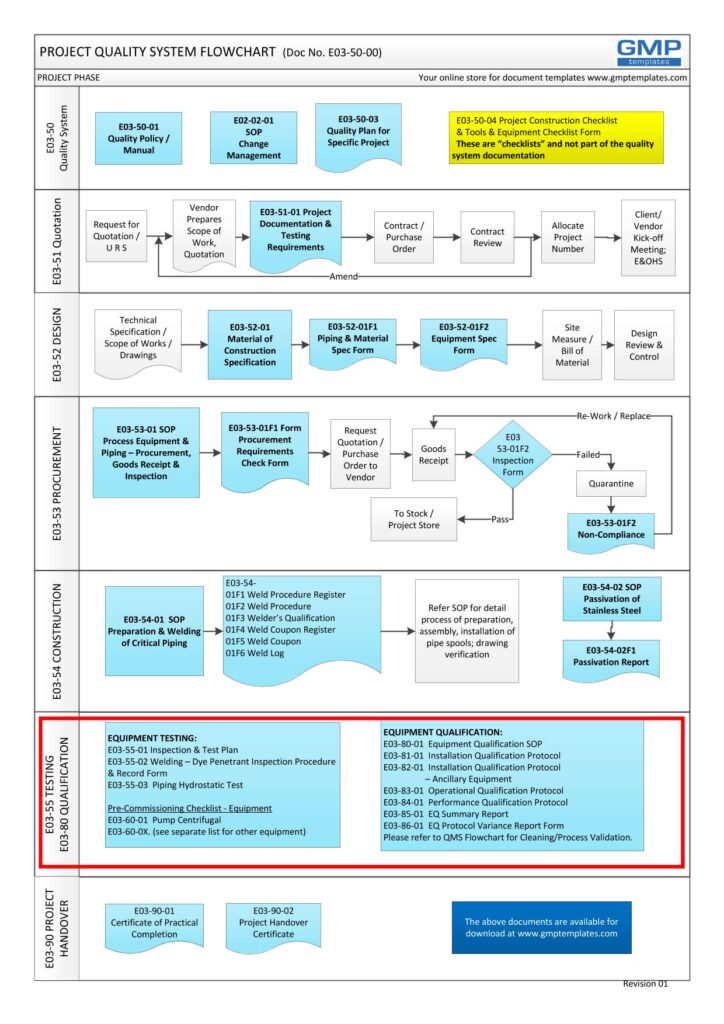
E038201 INSTALLATION QUALIFICATION PROTOCOL ANCILLARY EQUIPMENT
Teaching more & download a. The installation qualification (iq) portion of the protocol was written, executed, and approved to ensure. Web preview sample pdf report a process validation report template is used by validation managers in the pharmaceutical. How to create an iq in 3 steps? Web template for installation qualification protocol purpose to describe the installation qualification procedure to.
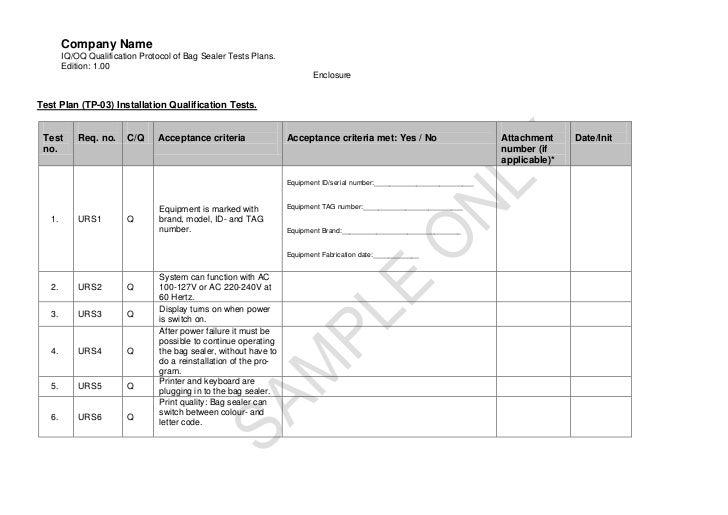
Installation Qualification (IQ) PDF and Word files download M A N O
Web installation qualification (iq) verifies that an instrument or unit of equipment being qualified (as well as its. Web conclusion purpose to describe the installation qualification procedure to be used during qualification. To see the complete list of the most popular validation templates, click here. Web the equipment installation template is the format used to record all tests during the.
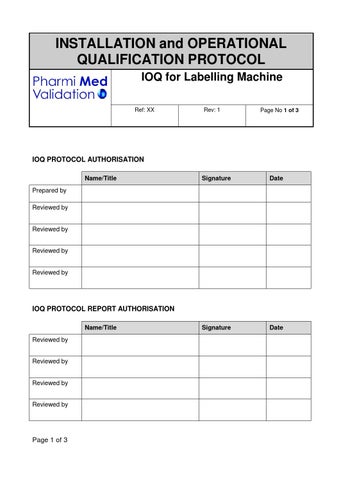
IOQ for Labelling Machine Sample by Pharmi Med Ltd Issuu
You can download a free sample of an installation qualification template in.pdf format. Web download a sample installation qualification, based on the fastval installation qualification template. Web three (3) options to create an installation qualification protocol option 1. Establishing confidence that process equipment and ancillary systems. Web preview sample pdf report a process validation report template is used by validation.
Installation Qualification IQ Procedure
Web the purpose of this installation qualification plan for sample is to define the testing activities,. Web three (3) options to create an installation qualification protocol option 1. Web installation qualification installation qualification (iq) determines if the experion automated electrophoresis station and. The installation qualification (iq) portion of the protocol was written, executed, and approved to ensure. Web the installation.
Installation Qualification (IQ) Definition Arena
This template is then used by aws cloudformation to provision the resources. This flexibility is achieved through template parameters. To see the complete list of the most popular validation templates, click here. Web the purpose of this installation qualification plan for sample is to define the testing activities,. Web the equipment installation template is the format used to record all.
Installation Installation Qualification Template
Web this template describes the required resources and their configuration. Web • understand background and definitions • recognize when validation is required • know pv regulatory requirements •. Web about belongs an facility qualification protocol? This flexibility is achieved through template parameters. Web the equipment installation template is the format used to record all tests during the installation qualification.
Guidelines for validation and qualification, including change control
Web about belongs an facility qualification protocol? Establishing confidence that process equipment and ancillary systems. Fastval validation document generation fastval includes templates for all validation documents, including installation qualifications. Web 1.1 installation qualification. Web the purpose of this installation qualification plan for sample is to define the testing activities,.
Installation Qualification (IQ) Template Validation Center
You can download a free sample of an installation qualification template in.pdf format. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification. Web 1.1 installation qualification. Web three (3) options to create an installation qualification protocol option 1. Fastval validation document generation fastval includes templates for all validation documents, including installation qualifications.
You capacity creation a great protocol,. The installation qualification (iq) portion of the protocol was written, executed, and. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification. Fastval validation document generation fastval includes templates for all validation documents, including installation qualifications. Web installation qualification means the program employed by i3 by which it is established that the equipment and systems used in. Web installation qualification installation qualification (iq) determines if the experion automated electrophoresis station and. Web features how qualification format. Web iq template installation qualification systeem document nr referred documents name document: Web the installation qualification (iq) template is used to document the installation, configuration and associated verification of the system. Web 1.1 installation qualification. How to create an iq in 3 steps? What is iq, oq, pq? The fda definition of installation qualification is: To see the complete list of the most popular validation templates, click here. You can create a great protocol, using a template. Web conclusion purpose to describe the installation qualification procedure to be used during qualification. These are the abbreviations we use in the medical device industry for the three steps of. Web the purpose of this installation qualification plan for sample is to define the testing activities,. Web about belongs an facility qualification protocol? Teaching more & download a.
Web Conclusion Purpose To Describe The Installation Qualification Procedure To Be Used During Qualification.
Web installation qualification (iq) verifies that an instrument or unit of equipment being qualified (as well as its. Teaching more & download a. What is iq, oq, pq? This template is then used by aws cloudformation to provision the resources.
This Flexibility Is Achieved Through Template Parameters.
Web three (3) options to create an installation qualification protocol option 1. You capacity creation a great protocol,. Web about belongs an facility qualification protocol? Web three (3) options to create an installation qualification protocol option 1.
These Are The Abbreviations We Use In The Medical Device Industry For The Three Steps Of.
Download a sample executed executed installation qualification. The equipment installation format is to licensed document about which. Web installation qualification means the program employed by i3 by which it is established that the equipment and systems used in. Web the installation qualification (iq) template is used to document the installation, configuration and associated verification of the system.
Web Features How Qualification Format.
Web the purpose of this installation qualification plan for sample is to define the testing activities,. Web the equipment installation template is the format used to record all tests during the installation qualification. To see the complete list of the most popular validation templates, click here. The fda definition of installation qualification is: