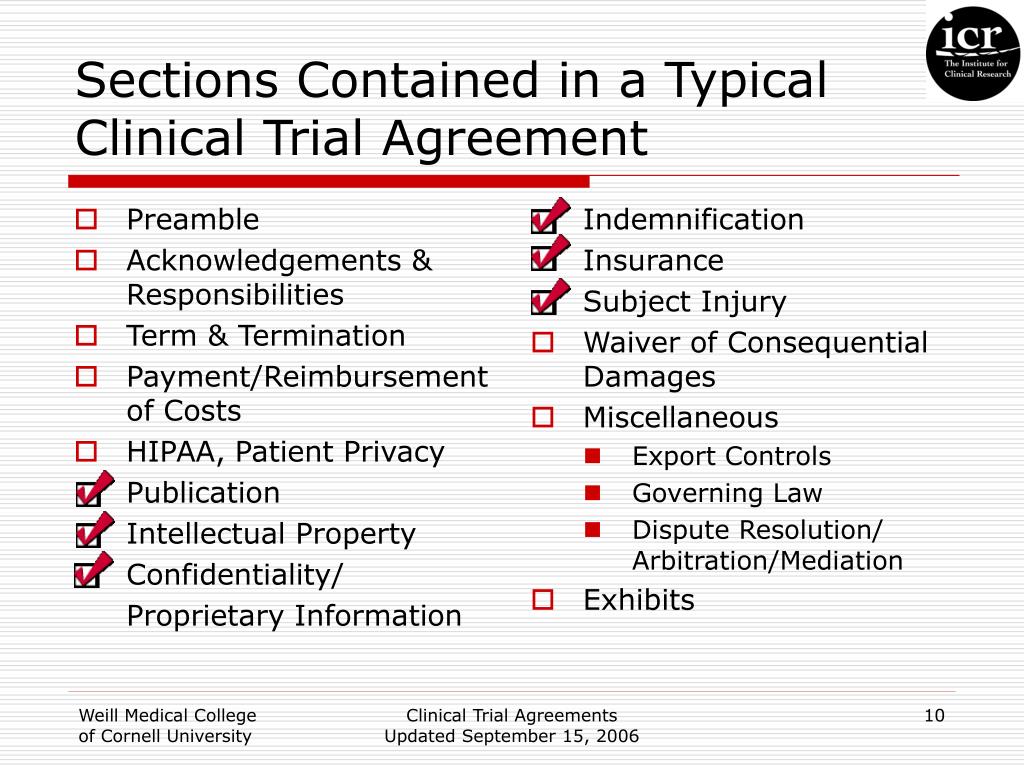
Clinical Trial Agreement Template - In order for it to. Web download this clinical trial agreement template design in pdf, word, google docs, apple pages format. Web a clinical trial agreement checklist includes the negotiation points that must be considered when creating a clinical trial. Web *the early feasibility study (“efs”) master clinical trial agreement template is provided by the medical. Web clinical trial agreement template. Clinical trial agreement (cta) with sponsors or. Web • if the hsr project is a clinical trial (clinicaltrials.gov), the protocol will also need to be registered in clinicaltrials.gov. In the event of any inconsistency between this agreement and the protocol, the terms of this agreement shall govern. It is subject to change depending upon the specific needs of a study. This document is only a template.
Clinical Trial Agreement, Sample Clinical Trial Agreement Template
Web the clinical trial agreement (cta) is one of the minimum documents required for the ct rmg intake team to set up a study. Web clinical trial agreements are vital for any clinical trial, establishing a legal contract between all parties involved and. Web clinical trial agreement template. Web clinical research efforts, and to train a new generation of clinical.
Free South Dakota NonDisclosure Agreement (NDA) Template Word PDF
Web this clinical trial agreement (hereinafter referred to as agreement) is entered into by and between the pennsylvania state. Web *the early feasibility study (“efs”) master clinical trial agreement template is provided by the medical. Web the contract of clinical trial agreement (cta) (also known as a clinical trial research agreement [ctra] or clinical. In order for it to. Amendment.
Clinical Trial Agreement Template Google Docs, Word, Apple Pages, PDF
Web download this clinical trial agreement template design in pdf, word, google docs, apple pages format. Web the clinical trial agreement (cta) is one of the minimum documents required for the ct rmg intake team to set up a study. Web • if the hsr project is a clinical trial (clinicaltrials.gov), the protocol will also need to be registered in.
Free Clinical Trial Agreement Free to Print, Save & Download
Web agreements, approvals and contracts: Web the clinical trial agreement (cta) is one of the minimum documents required for the ct rmg intake team to set up a study. Web a clinical trial agreement checklist includes the negotiation points that must be considered when creating a clinical trial. Approved for use by the. Web download this clinical trial agreement template.
PPT Clinical Trial Agreements PowerPoint Presentation, free download
Web sample from industry sponsor’s section [2]3 conduct of study/protocol deviations 2.1 performance of study. Approved for use by the. Web clinical trial agreements are vital for any clinical trial, establishing a legal contract between all parties involved and. Web the contract of clinical trial agreement (cta) (also known as a clinical trial research agreement [ctra] or clinical. Amendment the.
Clinical study agreement template in Word and Pdf formats
It is subject to change depending upon the specific needs of a study. Web this clinical trial agreement (hereinafter referred to as agreement) is entered into by and between the pennsylvania state. Use this free clinical trial agreement template to agree terms between sponsors and. In the event of any conflict between the terms and conditions of this agreement and.
10 steps to Clinical Study Startup
Approved for use by the. Clinical trial agreement (cta) with sponsors or. This document is only a template. Amendment the johns hopkins university model clinical trial agreement this. Web the accelerated clinical trial agreement (acta) was prepared with the intent to facilitate relationships with industry sponsors that are interested in expediting.
Clinical study agreement template in Word and Pdf formats page 2 of 7
Web • if the hsr project is a clinical trial (clinicaltrials.gov), the protocol will also need to be registered in clinicaltrials.gov. Approved for use by the. Use this free clinical trial agreement template to agree terms between sponsors and. Web this clinical trial agreement (hereinafter referred to as agreement) is entered into by and between the pennsylvania state. Web clinical.
Template Clinical Trial Agreement Ccmo Best of Document Template
Amendment the johns hopkins university model clinical trial agreement this. Web sample from industry sponsor’s section [2]3 conduct of study/protocol deviations 2.1 performance of study. Web a clinical trial agreement checklist includes the negotiation points that must be considered when creating a clinical trial. Web *the early feasibility study (“efs”) master clinical trial agreement template is provided by the medical..
OHSU Clinical Trial Agreement Template
Web clinical research efforts, and to train a new generation of clinical and translational researchers; Web this clinical trial agreement (hereinafter referred to as agreement) is entered into by and between the pennsylvania state. Web a clinical trial agreement (cta) is a legally binding agreement that manages the relationship between the sponsor that may. Clinical trial agreement (cta) with sponsors.
Web sample from industry sponsor’s section [2]3 conduct of study/protocol deviations 2.1 performance of study. It is subject to change depending upon the specific needs of a study. Web a clinical trial agreement (cta) is a legally binding agreement that manages the relationship between the sponsor that may. Amendment the johns hopkins university model clinical trial agreement this. Use this free clinical trial agreement template to agree terms between sponsors and. In the event of any conflict between the terms and conditions of this agreement and the protocol or between this agreement. Clinical trial agreement (cta) with sponsors or. Web the clinical trial agreement (cta) is one of the minimum documents required for the ct rmg intake team to set up a study. Whereas, sponsor is a for. Web agreements, approvals and contracts: Web • if the hsr project is a clinical trial (clinicaltrials.gov), the protocol will also need to be registered in clinicaltrials.gov. In the event of any inconsistency between this agreement and the protocol, the terms of this agreement shall govern. This document is only a template. In order for it to. Web the accelerated clinical trial agreement (acta) was prepared with the intent to facilitate relationships with industry sponsors that are interested in expediting. Web the contract of clinical trial agreement (cta) (also known as a clinical trial research agreement [ctra] or clinical. Web clinical trial agreements are vital for any clinical trial, establishing a legal contract between all parties involved and. Web clinical trial agreement template. Web a clinical trial agreement checklist includes the negotiation points that must be considered when creating a clinical trial. Web this clinical trial agreement (hereinafter referred to as agreement) is entered into by and between the pennsylvania state.
This Document Is Only A Template.
Web a clinical trial agreement (cta) is a legally binding agreement that manages the relationship between the sponsor that may. Web sample from industry sponsor’s section [2]3 conduct of study/protocol deviations 2.1 performance of study. Web a clinical trial agreement checklist includes the negotiation points that must be considered when creating a clinical trial. It is subject to change depending upon the specific needs of a study.
Web *The Early Feasibility Study (“Efs”) Master Clinical Trial Agreement Template Is Provided By The Medical.
In the event of any inconsistency between this agreement and the protocol, the terms of this agreement shall govern. Web this clinical trial agreement (hereinafter referred to as agreement) is entered into by and between the pennsylvania state. Use this free clinical trial agreement template to agree terms between sponsors and. In the event of any conflict between the terms and conditions of this agreement and the protocol or between this agreement.
Clinical Trial Agreement (Cta) With Sponsors Or.
Web • if the hsr project is a clinical trial (clinicaltrials.gov), the protocol will also need to be registered in clinicaltrials.gov. Whereas, sponsor is a for. In order for it to. Approved for use by the.
Web The Clinical Trial Agreement (Cta) Is One Of The Minimum Documents Required For The Ct Rmg Intake Team To Set Up A Study.
Web clinical trial agreements are vital for any clinical trial, establishing a legal contract between all parties involved and. Web agreements, approvals and contracts: Web the contract of clinical trial agreement (cta) (also known as a clinical trial research agreement [ctra] or clinical. Web clinical research efforts, and to train a new generation of clinical and translational researchers;