Clinical Evaluation Report Template - Performing data analysis for your medical device’s clinical evaluation Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device. Web the clinical evaluation plan defines methods for creating and updating the clinical evaluation report. Web the medical device regulation (mdr) applies from 26 may 2021. Of the 15 patients who did not have a clinical sample. Web clinical evaluation report template. Creating a process and establishing equivalency part 2: The clinical evaluation literature review process: Web clinical evaluation report sample. Web clinical evaluation report template:
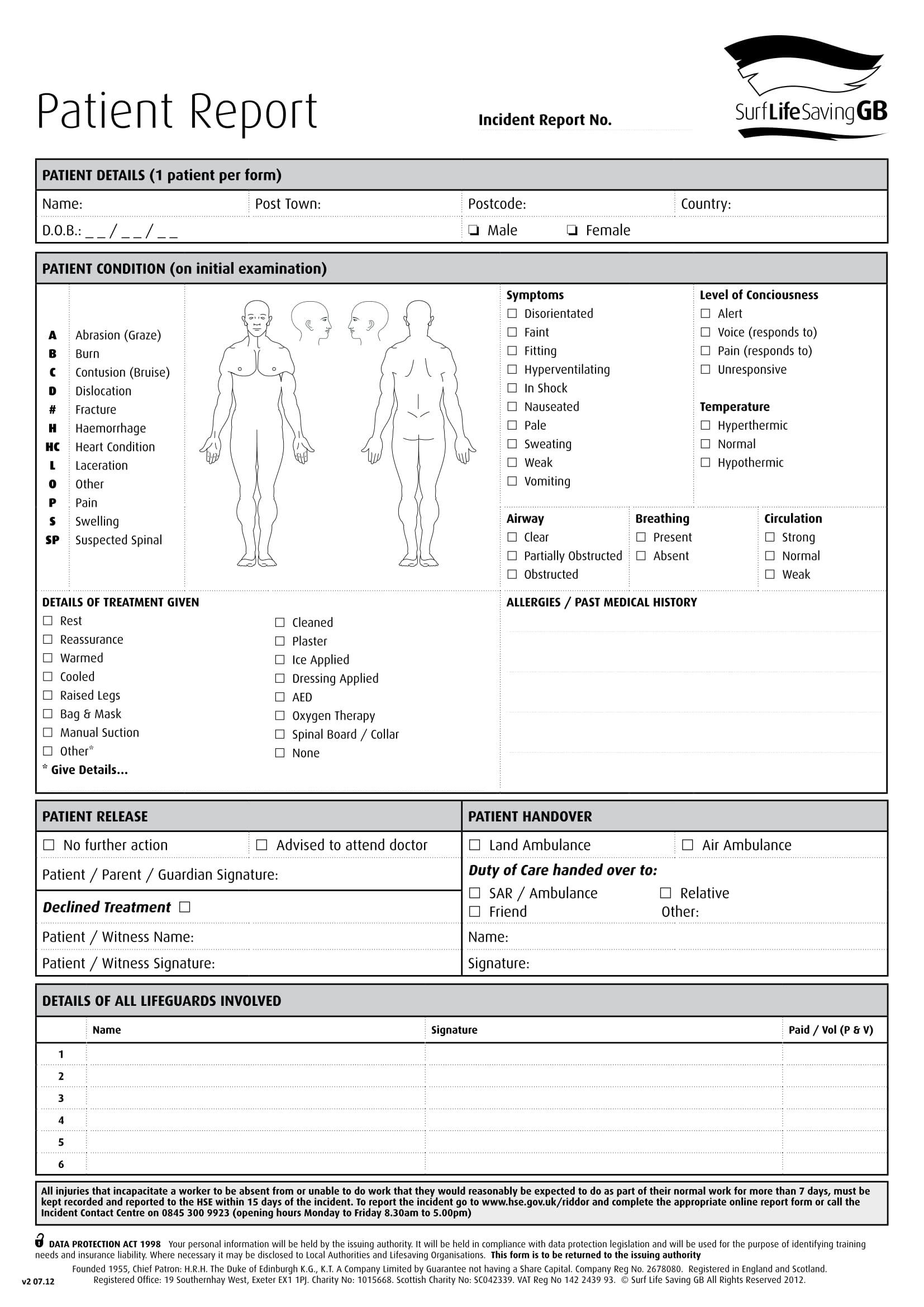
Patient Report Form Template Download Gambaran
Web a clinical evaluation assessment report (cear) template; A clinical evaluation report sample & template for medical device is a document that. Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device. Web the clinical evaluation plan defines methods for creating and updating the clinical evaluation report. Web clinical investigation application/notification documents:
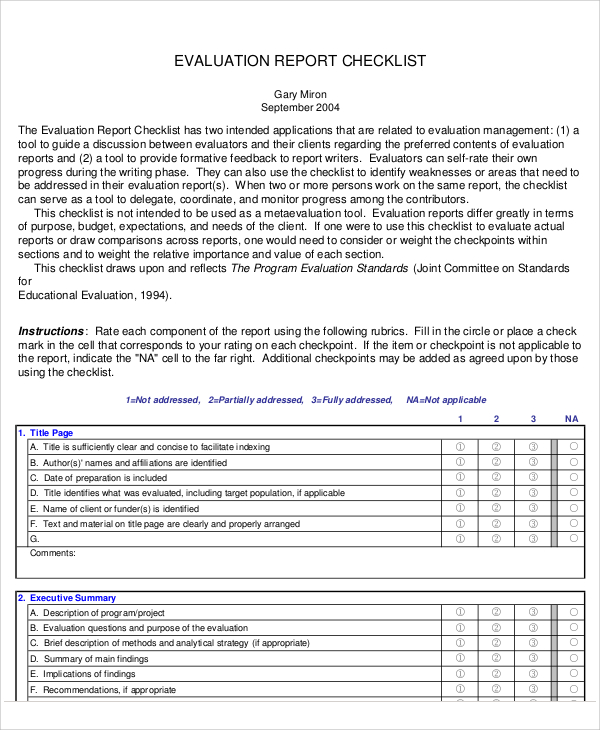
FREE 15+ Sample Evaluation Reports in PDF MS Word Apple Pages
Web our writing team has developed easy to use and edit clinical evaluation report templates, procedures and sop's. Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device. Web a clinical evaluation report (cer) provides clinical data to support a device’s clinical safety and efficacy. Web a clinical evaluation plan (cep) is a.
(PDF) Medical device clinical evaluation report (CER) rough template
Web 5+ clinical evaluation report templates 1. Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device. The in vitro diagnostics regulation (ivdr) applies. A free resource for medical device manufacturers news by kolabtree 13 jan. Web a clinical evaluation report (cer) provides clinical data to support a device’s clinical safety and efficacy.
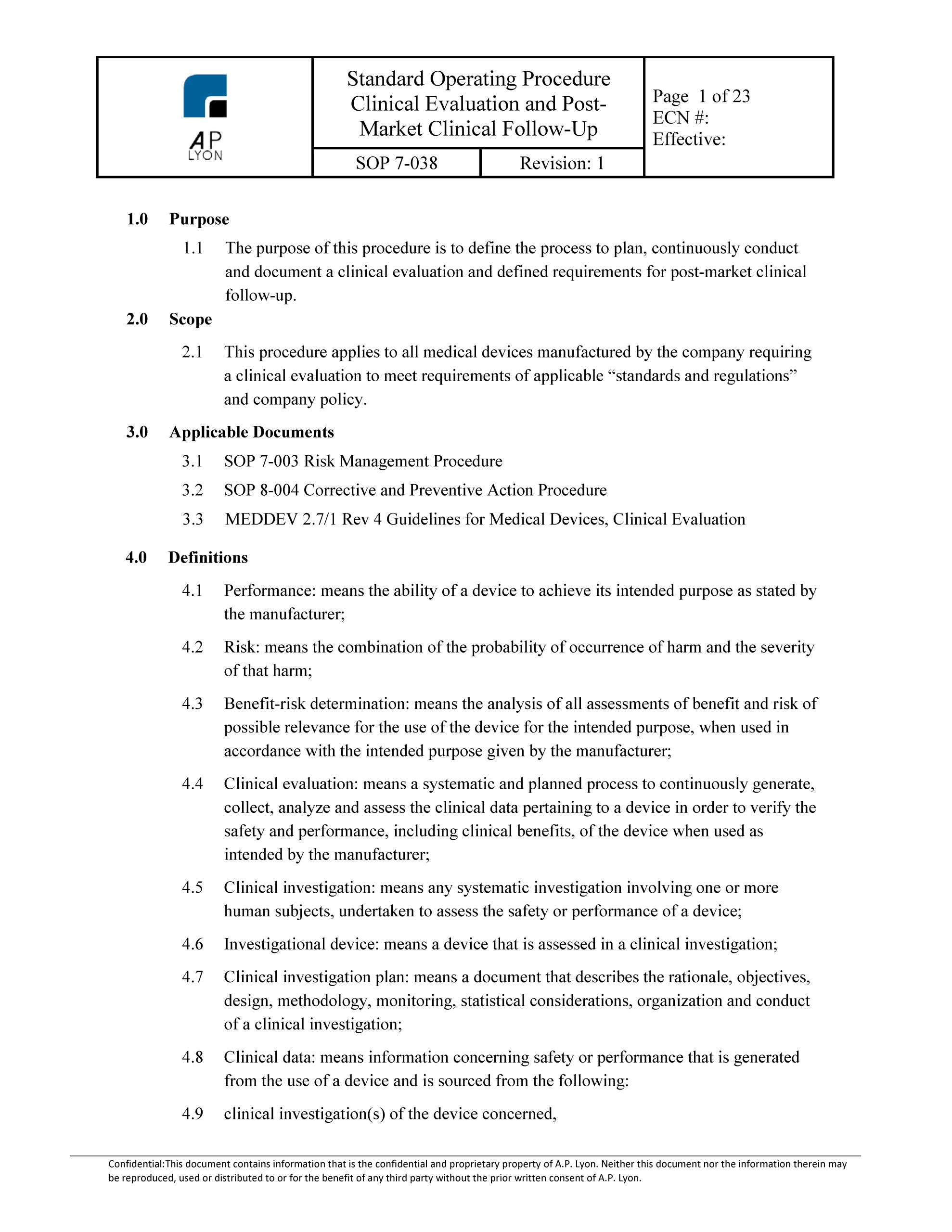
Clinical Evaluation Procedure
Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device. Clinical evaluation of a medical device: Web clinical evaluation report sample. Web clinical evaluation report template. Of the 15 patients who did not have a clinical sample.
sample evaluation report deped
The clinical evaluation literature review process: The in vitro diagnostics regulation (ivdr) applies. Web a clinical evaluation assessment report (cear) template; Web the clinical evaluation plan defines methods for creating and updating the clinical evaluation report. Identifying and appraising clinical data part 3:
Clinical Evaluation Procedure Bundle
Web a clinical evaluation report (cer) provides clinical data to support a device’s clinical safety and efficacy. Web clinical investigation application/notification documents: Web cer template guidance document apply a successful clinical evaluation process across your entire medical device. A free resource for medical device manufacturers news by kolabtree 13 jan. Web the clinical evaluation plan defines methods for creating and.
Clinical Evaluation Report Template Glendale Community regarding
Web cer template guidance document apply a successful clinical evaluation process across your entire medical device. Web a clinical evaluation assessment report (cear) template; Of the 15 patients who did not have a clinical sample. Creating a process and establishing equivalency part 2: Relevant data is collected and analyzed as part of writing the clinical evaluation.
Psychoeducational Report Template (4) TEMPLATES EXAMPLE TEMPLATES
Web a clinical sample was available for 44 patients. Performing data analysis for your medical device’s clinical evaluation Web clinical evaluation assessment report template, specifying recommended minimum content for a notified body clinical evaluation assessment. Web a clinical evaluation assessment report (cear) template; Identifying and appraising clinical data part 3:
Glory How To Write A Clinical Assessment Report What Does Subject
Web clinical evaluation report template: Web clinical investigation application/notification documents: Creating a process and establishing equivalency part 2: Web simply click the clinical evaluation report templates in this article such as customer clinical evaluation report examples,. Clinical evaluation of a medical device:
Clinical evaluationreport (CER) PEPGRA Healthcare
Web clinical investigation application/notification documents: A clinical evaluation report sample & template for medical device is a document that. Web a clinical sample was available for 44 patients. Web a clinical evaluation assessment report (cear) template; Web clinical evaluation assessment report template, specifying recommended minimum content for a notified body clinical evaluation assessment.
Performing data analysis for your medical device’s clinical evaluation Web clinical evaluation report sample. Web clinical evaluation report template: Of the 15 patients who did not have a clinical sample. The in vitro diagnostics regulation (ivdr) applies. Web a clinical evaluation plan (cep) is a detailed document that outlines the systematic approach and strategy for evaluating the. A clinical evaluation report sample & template for medical device is a document that. Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device. Web our writing team has developed easy to use and edit clinical evaluation report templates, procedures and sop's. Web simply click the clinical evaluation report templates in this article such as customer clinical evaluation report examples,. Web a clinical sample was available for 44 patients. Web report any financial or fiduciary interests that might appear to present a conflict of interest. Relevant data is collected and analyzed as part of writing the clinical evaluation. Web 1.2 clinical evaluation report. Web a clinical evaluation assessment report (cear) template; Web clinical investigation application/notification documents: Web 5+ clinical evaluation report templates 1. Web cer template guidance document apply a successful clinical evaluation process across your entire medical device. Identifying and appraising clinical data part 3: Web the medical device regulation (mdr) applies from 26 may 2021.
Identifying And Appraising Clinical Data Part 3:
A clinical evaluation report sample & template for medical device is a document that. Web clinical investigation application/notification documents: Web a clinical evaluation report (cer) provides clinical data to support a device’s clinical safety and efficacy. Web a clinical evaluation assessment report (cear) template;
The In Vitro Diagnostics Regulation (Ivdr) Applies.
Web clinical evaluation assessment report template, specifying recommended minimum content for a notified body clinical evaluation assessment. Web 5+ clinical evaluation report templates 1. Web the clinical evaluation plan defines methods for creating and updating the clinical evaluation report. Web our writing team has developed easy to use and edit clinical evaluation report templates, procedures and sop's.
Web The Medical Device Regulation (Mdr) Applies From 26 May 2021.
Web simply click the clinical evaluation report templates in this article such as customer clinical evaluation report examples,. Creating a process and establishing equivalency part 2: Web a clinical sample was available for 44 patients. Clinical evaluation of a medical device:
Relevant Data Is Collected And Analyzed As Part Of Writing The Clinical Evaluation.
Web clinical evaluation report template: A free resource for medical device manufacturers news by kolabtree 13 jan. Web a clinical evaluation plan (cep) is a detailed document that outlines the systematic approach and strategy for evaluating the. Web report any financial or fiduciary interests that might appear to present a conflict of interest.